Search
DePuy Failure Rates Much Higher Than Originally Thought. How Bad Will Things Get?
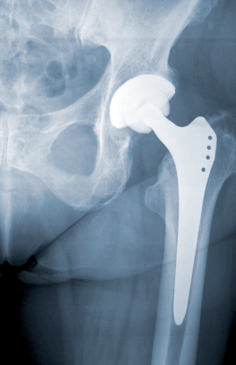 For almost a year now, people with DePuy artificial hips have been digesting the steady stream of news related to the problems associated with the hardware in their bodies. Now, new data seems to paint a far more dire situation than originally disclosed by the medical device manufacturer.
For almost a year now, people with DePuy artificial hips have been digesting the steady stream of news related to the problems associated with the hardware in their bodies. Now, new data seems to paint a far more dire situation than originally disclosed by the medical device manufacturer.
A recent statement released from The British Orthopaedic Association and the British Hip Society now estimates the failure rates of the DePuy ASR XL Acetabular System to be far higher than originally estimated.
By the groups’ calculations, the DePuy system failures may necessitate additional surgeries in 49% of people within six years after the system was put in place.
These figures are significantly higher than the DePuy failure rates reported by the National Joint Registry of England and Wales which estimated a failure rate of the ASR XL products to be 12% within the first five years of the surgery.
DePuy Lawsuits & Claims For Those Who Require Revisionist Surgery– And Those With Concerns About Their Future Medical Needs
More than 30,000 people in the United States have received DePuy hip replacement products that may be defective. Many of the recipients of the defective DePuy hips were younger– more active patients– who were steered towards the DePuy Products based on their perceived durability related to their metal-on-metal designs.
Though product failures have been seen in patients in all demographics, DePuy acknowledges some of most prevalent complaint likely come from smaller people and women who received the companies ASR head sizes less than 50 millimeters.
Product defects vary drastically in terms of how each person is impacted. However, common complaints related to DePuy ASR XL Acetabular System included:
- Pain or swilling in the hip
- Fractures
- Metal poisoning
- Hip dislocations
At this point, recipients of the DePuy ASR XL Acetabular System (which was recalled in August) likely have received correspondence from their surgeon or the medical center where the procedures were originally performed.
If you have received such letter, or believe that you indeed have received a DePuy hip replacement product in the past five years, you have legal rights regarding past problems and future medical monitoring that will likely be required for the extended future.
Given the relatively little information we have about the long-term impact of the DePuy ASR System, Nursing Home Law Center LLC encourages all recipients to speak with lawyers handling these matters. Based in Chicago, Nursing Home Law Center LLC is currently reviewing medical device cases involving DePuy ASR XL Acetabular System for individuals across the county.
For more information on nursing homes in Chicago look here. For laws related to Illinois nursing homes, look here.
Related:
J & J Hip Failure Rate as High as 49 Percent, U.K. Doctors Say, by Greg Farrell and David Voraecos, Bloomberg.com March 9, 2011
Defects With DePuy Hip Replacement Hardware May Give Rise To Lawsuits Against Manufacturer
In Aftermath Of DePuy Hip Problems, FDA Takes A Closer Look At Metal Hips
Johnson & Johnson’s Quality Catastrophe, Bloomberg Buisnessweek, March 31, 2011
 Nursing Home Law News
Nursing Home Law News